FDA Approved?
FDA Approved?
Actually, there is no such thing as an "FDA Approved" resin for 3D printing. The term "FDA Approved" is reserved for high risk drugs and medical devices which have been approved via a very stringent premarket approval (PMA) process that goes well beyond the standard FDA 510(k) pathway. Read the article below on the FDA website for this very important distinction:
What is the difference between FDA-listed, 510(k) exempt, cleared and approved medical devices? (fda.gov archived)
FDA "Exempt" Biocompatible Class 1 Dental Resins
Most 3D printer resins used in dentistry today are exempt from the FDA 510(k) process, although the FDA requires that the source specifically list them as exempt before marketing them. 3D Printed resins marked as FDA 510(k) Exempt have been tested by the manufacturer to be biocompatible and are listed by that manufacturer or distributor for tracking purposes. In this case, it is the manufacturer, not the FDA, that is claiming that these products are exempt from the FDA 510(k) process, usually because they make limited contact with the patient.
In general, FDA 510K Exempt 3d printer resins must use ingredients that have been tested for biocompatibility (ie. cytotoxicity and other ISO 10993 testing), and are thus considered to not present a high risk to the patient when used in most Class 1 and some Class 2 dental indications where exemption from the 510(k) requirements is allowed.
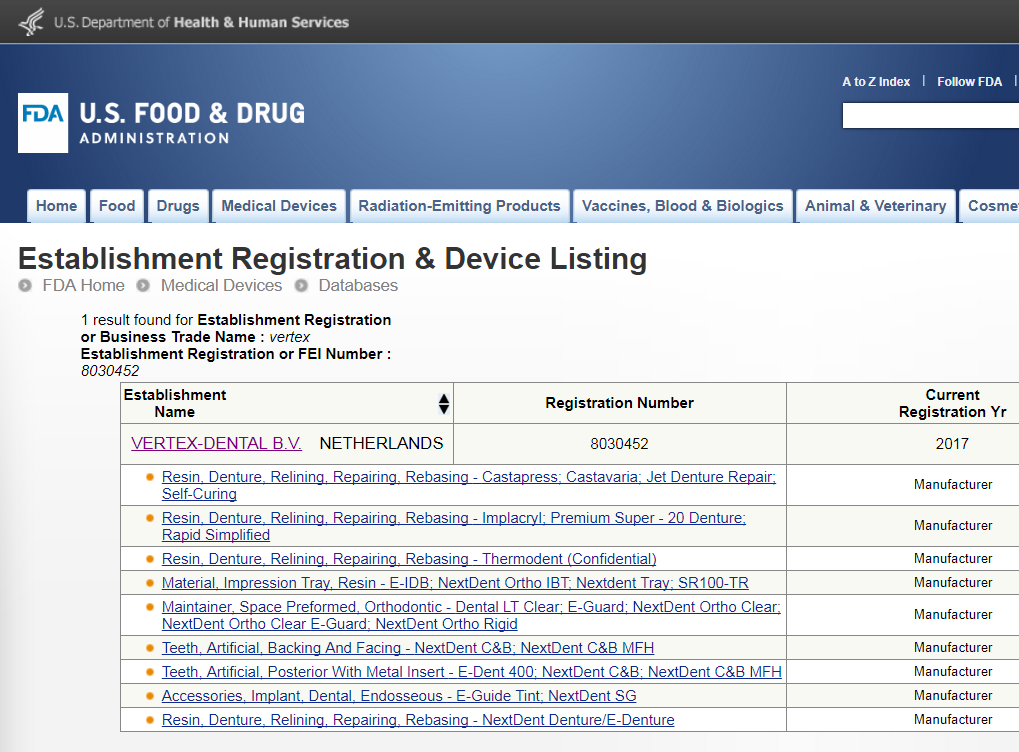
Updated Septebember 2017 - Click on the image below for current FDA listings for Vertex / NextDent materials
Disclaimer - Micron Inc is not affiliated with 3D Systems -- NextDent products are distributed in North America by Avadent.